

Since the question demands how many grams of NaCl must be added to 1000 g of water, we have to figure out the amount of water in the 20 percent of solution so that we can set up the correct conversion factor. The 20 percent NaCl solution means 20 g of NaCl/100 g of solution (read as 20 g of NaCl in a 100 g of solution). Of water to prepare a 20.0 percent NaCl solution. How many grams of NaCl must be added to 1000 g This means that the 20 g of NaCl/80 g of water can be used as a conversion factor to convert from mass of solute to mass of solvent and vice versa. So, if water was used as our solvent, our mass of water will be 80 g. Since mass of solution is equal to 100 g, we can get the mass of solvent by subtracting 20 g of solute from the 100 g of solution (100 g – 20 g = 80 g of solvent). For example, a 20.0 percent sodium chloride (NaCl) solution will contain 20.0 g of NaCl in 100 g of solution (20 g/100 g). Since the grams cancel out, the final unit will be in percent.
Formula for percent by mmass plus#
Notice! Mass of solution = mass of solute in grams plus mass of solvent in grams. Mathematically, % by mass = (mass of solute in grams/mass of solution in grams) x 100. Percent by mass (% m) is the mass of solute divided by the mass of solution multiplied by 100. If you have any difficulty in solving chemistry exercises, please leave a comment below and we will support you.How to calculate solution concentration in mass percent In order not to have difficulty in the exam and have the best results in studying, you should do many exercises to remember the formula longer and use the most accurate formula. The mass percent composition of N in NH 4NO 3 is:Ībove is the entire document on the formula for calculating mass percent.
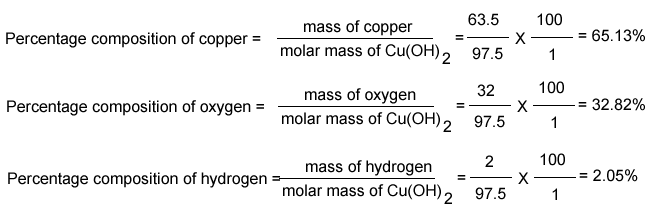
What is the percentage composition of nutrients in the fertilizer? Percentage of AL = (27.2,100)/102 = 52.94%Įxercise 2: A gardener used 350g of NH 4NO 3 to fertilize plants. The percentage composition by mass of the elements in the compound is: The molar mass of the given substance is: M AL2O3 = 23.2 + 16.3 = 102
Formula for percent by mmass how to#
= 11.18% Exercises with solutions on how to calculate mass percentĮxercise 1: Calculate the percentage smoke of the element present in the compound AL 2O 3? => Mass percent = (Molecular mass of the element/molecular mass of the compound) x 100 We find that the atomic mass of oxygen is 15.9994 and the atomic mass of hydrogen is 1.0079 Step 6: Substitute the variables into the mass percent equation.Step 5: Determine the mass of the element to calculate the mass percent.Step 4: Calculate the total mass of the compound.


Step 3: Multiply the atomic mass by the molar ratio.Step 2: Find the mass of each element in the compound (Look up the molecular weight of each element in the chemical formula on the periodic table).Instructions to solve the problem: To calculate this problem, apply the following 4 steps: If you don’t give mass, you can use molar mass to work out the mass percent of the elementĮxample: Calculate the mass percent of hydrogen in a water molecule.The units of these two values are g/mol.Mass percent = (mass of substance/mass of mixture) x 100Īnswer: The mass percent of 7g of sodium hydroxide dissolved in 100g of water is 6.542% Equation 2: Calculate mass percent when mass is unknownįormula for mass percent when mass is unknown = (molecular mass of element/molecular mass of compound) x 100 The problem gives sodium hydroxide is 7g, and water is 100g, so when dissolved, the mass of the mixture will be: 100g + 7g = 107gĪpplying the mass percent formula we have: Step 4: Apply the formula for calculating mass percent Step 3: Substitute the variables into that mass percent equation When the problem asks you to find the mass percent of a substance, you have to find the mass of that substance as a percentage of the total mass of all the ingredients.Step 2: Determine the mass of the substance to find the mass percent In case the mass of the compound or element is known, you can simply add them together to get the mass of the mixture/solution.Step 1: Calculate the mass of the mixture Instructions to solve the problem: To calculate the percentage of gas mass, you need to follow the following 4 steps Example: Calculate the mass % of 7g sodium hydroxide when dissolved in 100g water


 0 kommentar(er)
0 kommentar(er)
